Abstract
Infusions of hematopoietic stem cell (HSC) products can lead to several adverse events (AEs), including nausea/vomiting, hypo-/hyper-tension, allergic reactions, arrhythmia, encephalopathy and respiratory distress in HSC transplants (HSCT). These AEs have been attributed to toxicity of dimethyl sulfoxide (DMSO) and dead cells in patients who undergo HSCT with cryopreserved HSC products (Otrock et al, Transfusion, 2017). However, DMSO removal shows a limited effect on reducing AEs, and patient-specific factors, such as age and disease, also correlate with the incidence of AEs (Shu et al, BMT, 2014). Moreover, there are AEs associated with infusion of unfrozen HSC products, which have barely been studied.
Here, we prospectively surveyed AEs observed within 24 hours of infusion in all HSCT done in 16 transplant centers in Japan (UMIN000011646). From September 2013 to August 2016, a total of 1125 infusions, which were carried out in 570 [290 autologous (auto-) and 280 allogeneic (allo-)] peripheral blood stem cell transplants (PBSCT), 332 allo-bone marrow transplants (BMT) and 223 allo-cord blood transplants (CBT), were recruited. The 709 infusions in all auto-PBSCT and CBT, 195 allo-PBSCT and 1 BMT used cryopreserved products, whereas non-cryopreserved products were infused in the 416 HSCT that consisted of 85 allo-PBSCT and 331 BMT.
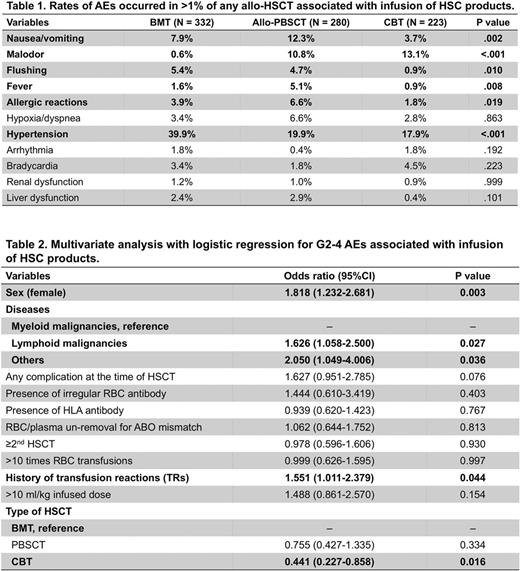
Unexpectedly, total incidences of AEs were more often reported in BMT (54.2%) and auto-/allo-PBSCT (49.8%), compared with CBT (39.0%, P= .001 and .008, respectively, by χ2 test with Bonferroni correction). Frequencies of total AEs were similar between auto- (49.7%) and allo- (50.0%) PBSCT, but grades 2-4 (G2-4) AEs (CTCAE 4.0) were more often noted in allo- (26.4%) than auto- (15.5%) PBSCT (P= .002). Thus, in allo-HSCT, G2-4 AEs were most often in BMT (37.7%) compared with allo-PBSCT (P= .004) and CBT (19.3%, P < .001). Comparing frequencies of G2-4 AEs in allo-HSCT using cryopreserved products (23.4%) with auto-PBSCT, there was a significant difference (P= .013). These findings suggest that some factors unrelated to cryopreservation, possibly including allo-antigens, contribute to AEs by infusions of HSC products. AEs that occurred in more than 1% of any allo-HSCT were nausea/vomiting, malodor, flushing, fever, allergic reactions, hypoxia/dyspnea, hypertension, arrhythmia, bradycardia, renal dysfunction and liver dysfunction (Table 1). Among them, hypertension was most often noted in BMT (P < .001), while nausea/vomiting ( P < .001), fever (P= .008) and allergic reactions (P= .019) were most frequent in allo-PBSCT. Life-threatening G4 AEs associated with infusions of HSC products were reported in 2 (0.18%) recipients: 1 CBT (anaphylaxis, hypoxia) and 1 allo-PBSCT with cryopreserved product (hypoxia, hypotension).
Subsequently, we sought risk factors that correlate with AEs. In univariate analysis for all HSCT containing both auto- and allo-HSCT, history of transfusion reactions (TRs, P= .002) and >10 ml/kg of infusion (P < .001) were noted as possible risk factors of AEs. Then, multivariate analysis with logistic regression identified that history of TRs was the only risk factor of AEs [odds ratio (OR) 1.459, P= .045], while >10 ml/kg of total infusion, as well as sex, disease, complications at the time of HSCT, presence of irregular erythrocyte antibody or anti-HLA antibody, plasma removal, past-HSCT history, >10 times of red blood cell transfusion, and types of HSCT did not show significance. In multivariate analysis exclusively for allo-HSCT, female sex, lymphoid malignancies versus myeloid malignancies, history of TRs, BMT versus CBT, were identified as risk factors of G2-4 AEs (Table 2). In addition, the rate of G2-4 hypertension was greater in BMT compared with allo-PBSCT (OR 2.793, P= .002) and CBT (OR 3.125, P= .002). On the other hand, >10 ml/kg of infusion (OR 12.058, P= .014) and PBSCT versus BMT (OR 8.755, P= .035) were correlated with G2-4 allergic reactions in allo-HSCT. We also found lower rates of allergic reactions in patients who received either ≤10 mL DMSO infusion (2.84%) or plasma-depleted HSC products (1.36%) compared with those given >10 mL DMSO (11.0%, P < .001) or plasma-intact products (5.58%, P < .037), respectively.
In conclusion, our data warrant the infusion risks of HSC products, which vary among the types of HSCT, highlighting the history of TRs and high infusion volume (>10 ml/kg) as risk factors for AEs.
Mori: Chugai Pharmaceutical Co: Research Funding; Pfizer Japan: Honoraria; Pfizer Japan: Research Funding; Bristol-Myers K.K: Research Funding; Novartis: Research Funding. Nagamura-Inoue: Tsubakimoto Chain CO.: Research Funding; Rohto Pharmaceutical Co., Ltd.: Research Funding; AMED grant: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal